Prostate matters is a not for profit organisation committed to providing free information about prostate issues from leading Clinical Authorities.
Home /
Innovations on the horizon


Overview by
Marcus Clark and St John Brown
Co Directors Prostate Matters
This page represents what we believe to be the most exciting Innovation in treating prostate disease developments in the diagnosis, treatment and management of prostate disease.
We are focusing on the following topics.
- Prostate cancer spit test better for men with high genetic risk than standard blood test. A spit test, where a sample can be collected at home, is more accurate at identifying future risk of prostate cancer for one group of men than the current standard blood test, a new study, BARCODE 1 reports.
- New Blood Test for Prostate Cancer Claims 94% Accuracy A new blood test for prostate cancer that offers greater accuracy than prostate-specific antigen (PSA) has been put to the test by researchers at the University of East Anglia (UEA) in collaboration with colleagues at Imperial College London (ICL) and King’s College London. They said that the test, developed by Oxford BioDynamics in collaboration with UEA, ICL, and Imperial College NHS Trust, could detect prostate cancer with 94% accuracy, significantly better than PSA testing.
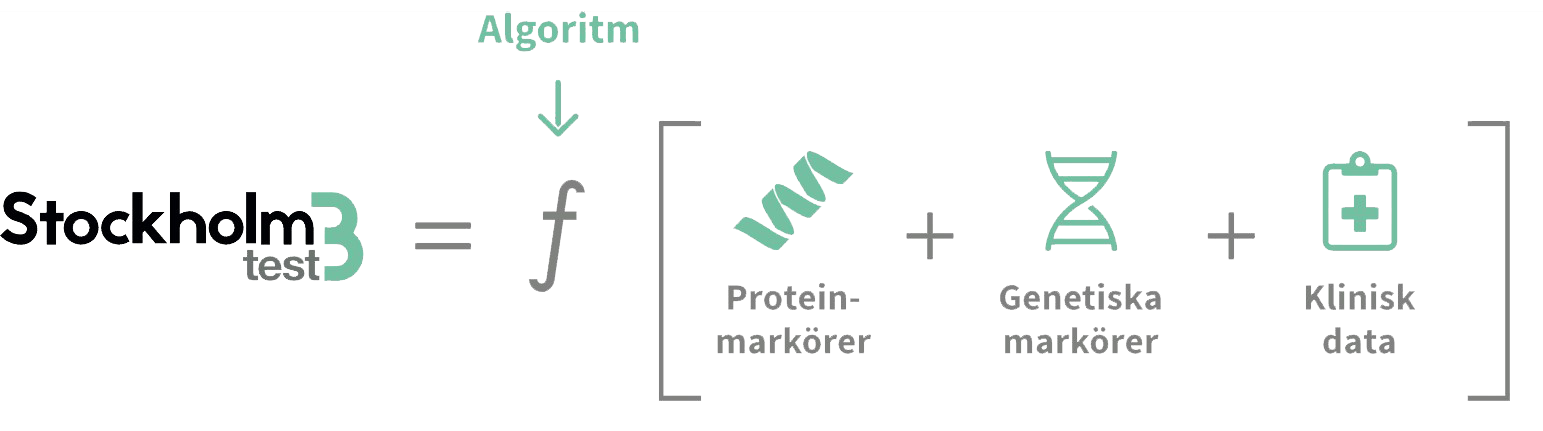
- There is a further blood test now available in the UK, Stockholm 3, which is far better than PSA and has been tested with 75,000 men
- The Prostogram trial which is pursuing the goal of a screening test for men which would be the equivelant of the mammogram screening for breast cancer and would revolutionise early detection and treatment.
- AI in detecting and reporting cancer from MRI. In this context Lucida Medical have developed Multi-stage AI analysis system to support prostate cancer diagnostic MR imaging
- New Focal Therapy technologies for minimally invasive treatment. As well as HIFU, Cryotherapy and Nano knife, there are several new technologies not yet in clinical use in the UK. These include, Francis Medical’s Transurethral thermal water vapour ablation, MR guided transurethral ultrasound ablation (TULSA -PRO) for cancer and BPH from Profound Medical and Focal Laser Ablation (FLA) of prostate cancer. Finally there is another laser treatment now in use produced by Elesta, Echolaser Thermal Ablations. It is available in Ireland but not yet in the UK. Their is a link to the latest outcomes study for laser treatmemts which is very encouraging.
- Benign Prostatic Hyperplasia. A new trial testing a drug coated balloon catheter.Following a successful pilot study, the ongoing randomized PINNACLE trial is aiming to confirm the safety and efficacy of the Optilume. Results from the EVEREST-1 pilot study (NCT03423979) presented at the 2020 American Urological Association Virtual Experience showed that Optilume is safe and provides significant improvements in BPH-related lower urinary tract symptoms.


Researchers at The Institute of Cancer Research, London, and The Royal Marsden NHS Foundation Trust trialled the new test, which looks for genetic variants linked to prostate cancer.

The researchers set out to assess the accuracy of the Prostate Screening EpiSwitch (PSE) test, which uses 3D genomics to detect cancer-specific chromosome conformations in a person’s blood sample. Their study, published in Cancers, aimed to determine whether combining the EpiSwitch test with traditional PSA testing would increase overall diagnostic accuracy.

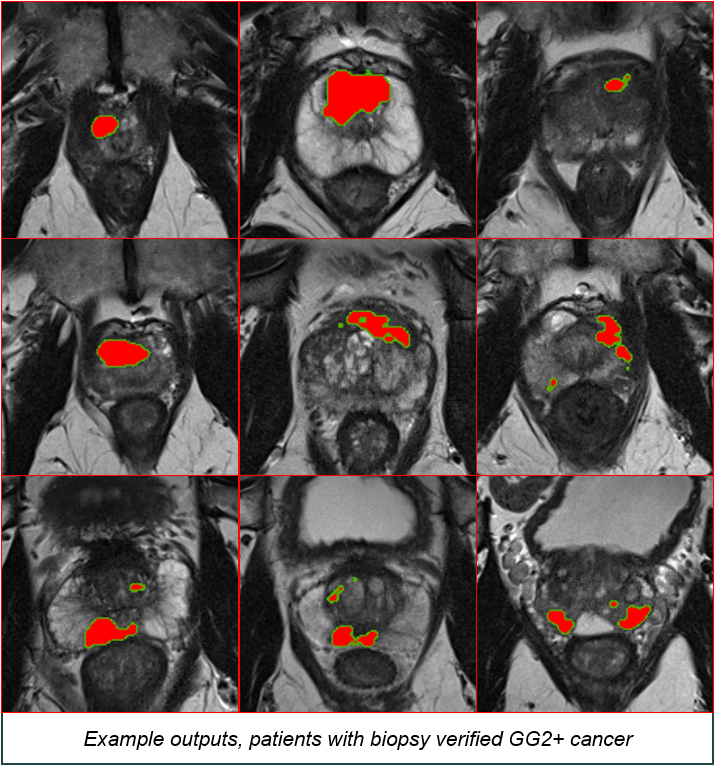
Lucida Medical have developed Multi-stage AI analysis system to support prostate cancer diagnostic MR imaging
As illustrated by the following graph:
These example outputs show patients with a biopsy verified Gleason score >=3+4 cancer
On 1 st May 2024, a landmark prostate cancer screening study was announced by Prostate Cancer UK: the TRANSFORM trial. The study is part of a major collaborative effort between some of the leading prostate cancer researchers in the UK and will be led by Professor Hashim Ahmed at Imperial College London.
The government-backed trial is supported by a £42 million grant from Prostate Cancer UK and co-funded by the National Institute for Health and Care Research, with support from a number of other prostate-focused organisations.
What is it?
TRANSFORM is one of the largest ever screening studies for prostate cancer and will involve over a quarter of a million men who will be followed up for over 10 years. The aim of the study is to find out the best way to screen men for
prostate cancer and in doing so, reduce the number of men dying from the disease. An initial pilot phase of the study will take place over three years and involve approximately 12,500 men. It will test whether standard prostate MRI
scans or new, shorter MRI scans can detect cancer in men from the general population who have no specific symptoms of prostate cancer. It will specifically look at the effect of MRI in men who have a normal or raised PSA blood test. The pilot will also review the impact of genetics, using simple blood tests called polygenic risk scores. This first phase of the study will help the researchers decide on the best screening tests to use in the main study.
It is expected that the study will run for at least 15 years to find out if screening can reduce deaths from prostate cancer. Importantly, it will also aim to find out which tests come with the least harm and are able to diagnose the most
dangerous cancers, without finding the low-risk cancers that can never impact patients. The team behind TRANSFORM expect it to be highly flexible so that if new screening tests become available, they can also be included in the trial.
Certain groups, such as men with weat african genetic heritage, which includes Afro Cabibeann men, are known to be at greater risk of dying from prostate cancer than the general population. As a result, the TRANSFORM trial
will specifically reach out to black men to ensure that they are well-represented in the study. By collecting samples, images, and data from over 250,000 men, TRANSFORM will also help build one of the largest prostate cancer biobanks in the world which will become available to researchers to help guide major new
discoveries.
Why do we need TRANSFORM?
There is currently no formal screening programme for prostate cancer in the UK. At present, the NHS website recommends men to see their GP if they have symptoms, at which point the GP may perform a prostate examination involving a finger in the back passage (DRE), as well as a PSA test. This pathway has several flaws that may lead to the most dangerous cancers being missed. Firstly, prostate cancer has no symptoms in the vast majority of cases. Secondly, and most importantly, the PSA test is highly inaccurate. This is discussed in more detail on the “Screening for Prostate Cancer” page. In summary, the PSA test can miss life-threatening cancers (a false negative test) or can be raised when no cancer exists (a false positive test). False positive tests mean that men undergo invasive prostate biopsy for no reason. Worryingly, the PSA test can also be raised when low-risk cancers are present. These low-risk cancers are of such low likelihood to spread outside of the prostate that some urologists and pathologists debate whether they should even be called cancer! Unfortunately, some men with low-risk cancers will be treated when they don’t need to be: this is called overtreatment. Overtreatment is dangerous because treatments such as surgery or radiotherapy can come with significant side effects like erectile dysfunction and incontinence (leaking urine).
Large screening studies in the past have shown that PSA screening can reduce the number of deaths from prostate cancer by approximately 20%. But due to the serious risks of missing dangerous cancers and overtreating low-risk cancers, the UK National Screening Committee voted against screening with the PSA test alone in 2020.
TRANSFORM aims to change this. It is hoped that the new screening tools testing in TRANSFORM will increase the detection of life-threatening cancer whilst reducing unnecessary invasive biopsies and detection of low-risk cancers (and with that, reduce overtreatment).
When will it start?
Planning is already underway. The first phase of TRANSFORM will begin later this year with the first group of men invited to join in late 2024/early 2025.
Am I eligible for the trial?
If you are between the age of 50 and 75, or 45 for Black men, then you may be eligible. You won’t be able to volunteer for the trial, but letters of invitation will be sent out to eligible men via GP practices. TRANSFORM could save the lives of thousands of men every year. If you want to be part of this revolutionary study then please do respond to your letter of initiation when it arrives!
For more information visit the Prostagram page:
Read more about Lucida AI:
More useful links:

Prostate matters is a not for profit organisation that is committed to providing free expert advice about prostate issues from leading Clinical Authorities
In memory of Riki
PROSTATE MATTERS
Copyright Disclaimer: We try to acknowledge copyright as appropriate. If we have used something without acknowledging copyright, this is inadvertent. Please let us know by emailing info@prostatematters.co.uk
Site design and technical development by Webtoys | Intelligent Digital Media

