Prostate matters is a not for profit organisation committed to providing free information about prostate issues from leading Clinical Authorities.
Proclarix, a blood test for prostate cancer risk

Overview by Professor Chris Booth
Retired Consultant Urological Surgeon
and
Clinical Director of the CHAPS charity
Summary
Proclarix is a non-invasive blood test which analyses protein biomarkers to assess the risk of aggressive prostate cancer,
It is particularly useful for those men with a PSA between 2 and 10 ng/nL. This is referred to as the PSA “grey area”, where it’s unclear if a raised PSA level indicates prostate cancer or not.
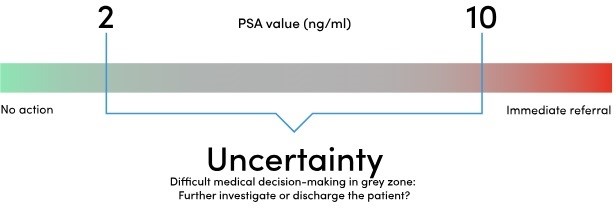
A further, a PSA within these “normal” ranges doesn’t rule out prostate cancer – 1 in 7 men with prostate cancer have a normal PSA.
Below is a table which illustrates typical ‘normal’ PSA ranges by age
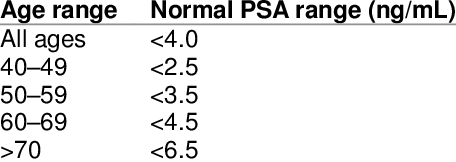
However this table is for ‘guidance’ only. It is recommended you read the page on what is normal and abnormal PSA.
Proclarix provides clarity on these unclear PSA results by giving a more accurate and specific risk score for aggressive prostate cancer. A high-risk Proclarix score would indicate that you should go to the next stage of investigation – MRI. A low-risk score rules out prostate cancer with 95% accuracy. Proclarix can be used instead of a standard PSA blood test with no additional intervention required, with results available within 5 to 7 days.
Below the button below to see an example report.
How does Proclarix work?
Proclarix is a blood test that measures protein biomarkers in the blood, including: Thrombospondin 1 (THBS1), Cathepsin D (CTSD), Serum total PSA (tPSA), and Free PSA (fPSA)
A software algorithm then combines these measurements with the patient’s age to generate a risk score. A high-risk score indicates that the patient should undergo further investigation, such as an MRI, while a low-risk score rules out prostate cancer with 95% accuracy.
The evidence
For those interested in the technicalities of clinical evidence, Proclarix has been CE-IVD-labeled based on the retrospective analysis of 955 subjects collected from two different hospital cohorts. All subjects had a total PSA 2-10 ng/ml, a normal DRE, enlarged prostate volume, and a confirmatory TRUS-guided prostate biopsy. In this analysis, at 90% sensitivity, the specificity was 43% (versus 18% for %fPSA), with a negative predictive value of 95% for detecting a clinically significant prostate cancer, i.e., a Gleason score ≥7

Prostate matters is a not for profit organisation that is committed to providing free expert advice about prostate issues from leading Clinical Authorities
In memory of Riki
PROSTATE MATTERS
Copyright Disclaimer: We try to acknowledge copyright as appropriate. If we have used something without acknowledging copyright, this is inadvertent. Please let us know by emailing info@prostatematters.co.uk
Site design and technical development by Webtoys | Intelligent Digital Media

